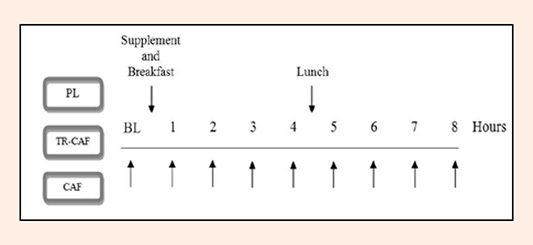
Figure 1. Study Protocol. The experimental trials were randomized into one of three trials: placebo (PL); time-release caffeine supplement (TR-CAF); and a regular caffeine supplement (CAF). Participants reported following an 8-hour fast and were instructed not to exercise 24 hours prior. Assessments took place at baseline (prior to supplement ingestion) and at each hour following ingestion of the supplement for a total of 8 hours. Assessments consisted of blood measures, metabolic measures, cardiovascular measures, subjective measures, and performance measures. Between assessments, participants sat comfortably in a quiet room without distraction wearing noise cancelling headphones and were provided a standardized breakfast and lunch.