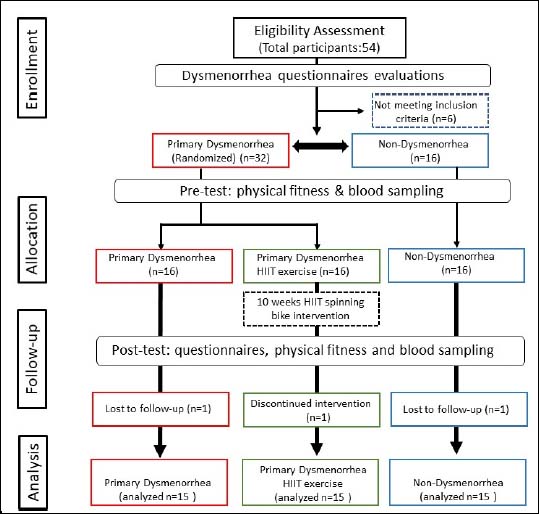
Figure 2. Study flow diagram. This study is a randomized, parallel-controlled clinical trial. There were 48 eligible participants, who were randomly assigned into three groups, allocated as Non-Dysmenorrhea (Control), Primary Dysmenorrhea (Dysmen), and Primary Dysmenorrhea undergoing HIIT exercise (DysmenHIIT). Dysmenorrhea was defined by menstrual pain intensity, measured using the VAS method in the SF-MPQ. The questionnaires and physical fitness were assessed before and after the experimental intervention. Blood samples were taken three days before menstruation and biochemical variables and hormones associated with menstrual pain were analyzed before and after the intervention.