|
|
|
| |
| ABSTRACT |
|
The purpose of this study was to determine the effects of a mechanical loading course (short-term free-fall landing) on femoral geometry and biomechanical properties in growing rats. Thirty-two female Wistar rats (7-week-old) were randomly assigned to three groups: L30 (n = 11), L10 (n = 11) and CON (n = 10) groups. Animals in the L10 and L30 groups were subjected to a 5-day free-fall landing program in which animals were dropped from a height of 40cm 10 and 30 times per day, respectively. Landing ground reaction force (GRF) was measured on the 1st and 5th days of landing training. All animals were subjected to two fluorescent labeling injections on the days before and after the 5-day landing training. Three days after the last labeling injection, animals were sacrificed under deep anesthesia. Methods of dynamic histomorphometry, tissue geometry and tissue biomechanical measurements were used to investigate the response in femora. A significant decrease in peak GRF in the hind-limb was shown from day 1 to day 5. No significant difference was shown among groups in dynamic histomorphometry. Biomechanical property analyses showed significantly lower maximal energy and post-yield energy in the L10 and L30 groups as compared to the CON group (p < 0.05). Moreover, geometric measurements revealed that cross-sectional cortical areas and thicknesses were significantly lower in landing groups than in the CON group. Short-term (5-day) free-fall landing training resulted in minor compromised long bone tissue, as shown by reduced bending energy and cortical bone area but not in other mechanical properties or tissue measurements (e.g. weights and length) of growing female rats. Further studies would be valuable to investigate whether this compromised bone material represents the existence of a latency period in the adaptation of bone material to external mechanical loading. |
| Key words:
Bone, mechanical load, biomechanical properties, post-yield energy, animal model
|
Key
Points
- Short-term free-fall landing causes compromised bone material as shown by reduced post-yield energy in long bones of rodents.
- The results of the current study suggest the existence of unsettled bone material after a short-term mechanical loading regime.
- The connection of the present animal study to the stress fractures occurring in young athletes needs to be clarified.
|
Participation in physical activities plays an important role in peak bone mass accumulation and bone health during developmental periods (Pitukcheewanont et al., 2010). According to previous studies, physical activity participation is not always positive for bone health. Exercise training (e.g. distance running) sometimes negatively impacts bone mass and can increase the risk of stress fracture (Harrast and Colonno, 2010; Warren and Chua, 2008). Nevertheless, it has been mentioned that doing high-impact or weight-bearing exercise during growth periods can provide benefits for bone mass and bone strength (Daly, 2007; Hind and Burrows, 2007), and some of those benefits might be retained and be helpful for reducing fragility fractures in old age (Karlsson, 2007). Many human and animal studies have suggested that a period of high-impact exercise training will enhance bone size (Kato et al., 2009), bone mineral density (Scerpella et al., 2003) and bone strength (Umemura et al., 1997; Umemura et al., 2008; Welch et al., 2008) for young growing bone. However, the mechanisms and procedures through which bone adapts to mechanical loading need further clarification. For instance, changes in various biomechanical properties or dimensions of bone tissues corresponding to the external mechanical stress have not been well investigated. Animal models can be used for obtaining more mechanistic information, as well as for detailing procedures regarding bone’s reaction when subjected to mechanical stimuli. In the past decades, substantial evidence has already been provided by animal studies using jumping and/or free-fall landing as an exercise mode for promoting bone formation, strength, mass or size in young growing animals (Judex and Zernicke, 2000; Umemura et al., 1997; Umemura et al., 2008; Welch et al., 2008; Welch et al., 2004). However, when compared to a standard bone remodelling cycle in rats (e.g. 16 days) (Tran Van et al., 1982), those previous studies looked at the results of relatively long-term (e.g. 8 weeks or longer) experimentation. Only two previous studies investigated the effects of short-term free-fall landing (Lin et al., 2011) and jumping (Nagasawa et al., 2008) on fore- and hind-limb bones, respectively. Lin and colleagues (Lin et al., 2011) verified that a 5-day free-fall landing training (10 or 30 times/day) would enhance cortical bone formation activity and bending energy dissipation in rodent ulnae without changes in bone size. Nagasawa and colleagues also showed that cortical bone formation activity was enhanced after a three-session (with training on days 1, 3 and 5) jumping training, but no difference was revealed in the mechanical properties of tibiae (Nagasawa et al., 2008). However, in Nagasawa’s study, biomechanical measurements included only the maximal bending force of tibiae. According to previous studies, there are several other biomechanical parameters, such as post-yield behaviors, valuable for estimating bone’s mechanical properties. Recently, it has been suggested that post-yield behaviors of bone tissue are associated with the quality of bone matrix (e.g. collagen) (Burr, 2002; Garnero et al., 2006; Nyman et al., 2007; Skedros et al., 2006). Two previous studies verified that a short training period (3 weeks) of treadmill running can increase post-yield displacement of hind-limb bones (Wallace et al., 2007; Wallace et al., 2009). In addition, our previous work demonstrated that a 5-day high-impact exercise training (free-fall landing) could also enhance the post-yield energy absorption in fore-limb bone (e.g. ulnae). Since most of the relatively long-term exercise studies, which used different training models (e.g. treadmill running, jumping and landing), focus on the adaptations of hind-limb bones, examining similar bones after a shorter period of high-impact exercise could help expose the mechanisms through which those adaptations occur. According to those previous short-term studies (e.g. jumping, landing), the biomechanical properties of bone tissues should be sensitive in responding to external mechanical loading. The purpose of this study was to determine the effects of a short-term free fall landing protocol on hind-limb bones in growing rats. In addition to using various biomechanical measurements, we also evaluate the effects of free-fall landing using dynamic histomorphometry and geometric measurements. AnimalsFemale Wistar rats (n = 32) were housed under controlled conditions including a room temperature of 22 ± 1°C and a 12:12 hour light-dark cycle. All of the animals were fed with Purina Rodent Chow5001 (Labdiet®, Richmond, IN, U.S.A.) containing 0.95% calcium and 1.07% phosphate (wt/wt of dry food) and tap water ad libitum. The procedures of the current animal study were approved by the Committee of Animal Study at National Cheng Kung University (No. 940054) and the “Principles of laboratory animal care” (NIH publication No. 86-23, revised 1985) were followed. During the training periods, all animals were healthy and free from any infection, and the body weight (BW) of each animal was measured daily.
Experimental designThe exercise training programs began when the rats reached an age of seven weeks. Animals were randomly assigned to three groups: 1) the L30 group (n = 11), animals subjected to 30 free-fall landings per day, 2) the L10 group (n = 11), animals subjected to 10 free-fall landings per day, and 3) the CON group (n = 10), a sedentary control group. Animals in the landing groups were gently held and released from a height of 40 cm, with four limbs simultaneously landing on a dry flat surface (Lin et al., 2011). A ≥ 10-second interval was arranged between each landing. The CON group maintained normal activity in the cages. In the current study, animals in the landing groups were subjected to a 5-day training period. All animals were subjected to two fluorescent labeling injections; one on the day before (calcein, 8 mg·kg-1 b.w.) and one on the day after (tetracyclin, 20 mg·kg-1 b.w.) the landing training period. Three days after the second fluorescent labeling injection, animals were sacrificed under deep anesthesia (sodium pentobarbital, i.p. injection, 65 mg·kg-1 body weight). For the time schedule of the current animal experiment, please refer to Figure 1.
Ground reaction force (GRF) measurementThe force platform (30cm 30cm) in the current study was designed by our laboratory, which included four load cells (10 lbs., MDB-10, Transducer Techniques®, Temecula, USA), an amplifier (InstruNet-100, GW Instruments, Inc., Somerville, USA ) and a data acquisition card (InstruNet-230, GW Instruments, Inc., Somerville, USA) connected to a personal computer with a sampling rate at 100 Hz. The calculation of hind-limb GRF was based on the concept of force equilibrium for a force platform, which was similar to that used in a previous study (Welch et al., 2009). Original landing data (unit: N) of the five initial free-fall landings for each rat were obtained from four load cells on the 1st and 5th days of the training program. In order to further calculate the GRF on the hind-limbs, the landing spots of the four paws were recorded using a video camera set up above the platform. Briefly, a snapshot of each landing on the force platform was saved from the video files. By setting a specific load cell as the origin of an xy coordinate graph, the position of each paw was converted to a coordinate (x, y) on the platform. Then, peak vertical GRFs were calculated by combining the peak landing values of load cells with the coordinates of paws using a self-built program in Matlab 6.5 (The MathWorks, Inc., Natick, USA). The GRF value of each rat was the average of contra-lateral legs and revealed as folds of total body weight, which were calculated. For calculation details, please see Appendix. Coefficients of variations (%) for the landing GRF values of each rat were calculated from its 5 trials. The CVs for the two landing groups (L10 vs. L30) on the first day and the fifth day were 27.0 % vs. 18.3 % and 23.8% vs. 23.3 %, respectively.
Bone sample preparationThree days after the second fluorescent labeling injection, all animals were sacrificed by decapitation under deep anesthesia (sodium pentobarbital, i.p. injection, 65 mg/kg). Right femora were harvested, and soft tissues were removed, wrapped in normal saline-soaked gauze and aluminum foil, and stored at -20 °C for the biomechanical analysis. Before biomechanical testing, femora were thawed at room temperature and weighed as wet weight (WW). After the completion of biomechanical testing, the fractured bone specimen were immersed in a solvent (2 volumes of chloroform combined with 1 volume of methanol) for 1 week and then dried at 80 °C for 24 h (Honda et al., 2001). Fat-free dry weight (FFDW) of each femur was measured after the 24 h drying period. Subsequently, the breaking site of each defatted and dehydrated femoral tissue was gently ground to a flat surface perpendicular to the long axis of each femur and subjected to cross-sectional photography (please see details in the next section). After the cross-sectional photography, all bone tissues were subjected to methymethacrylate (MMA) embedding.
Biomechanical testing and geometry measurementUsing the methods of a previous study (Huang et al., 2003), a three-point bending test was performed in the anteroposterior (AP) direction (posterior surface in tension) of femora using a materials testing system (MTS-819, MTS System, Minneapolis, MN, USA). The span of the two support points was 20 mm, and the deformation rate was 1 mm/sec. Load/deformation data were transported to a personal computer and acquired by Team 490 software (Version 4.10, Nicolet Instrument Technologies Inc., Madison, WI, USA). Load-deformation data were used for further calculating various extrinsic (whole bone) mechanical properties, including yield load, maximal load, fracture load, yield load energy, post-yield load energy, maximal load energy, fracture load energy, and stiffness. Using a digital charged coupled device (CCD) camera (Moticam 2300, Motic Instruments, Inc., Richmond, BC, Canada), photographs (resolution: 1024 X 768 pixels) of the failure sites were taken using a modified process (Huang et al., 2003) on the ground surfaces (mentioned above). Cross-sectional parameters, including total cross-sectional area, cortical bone area, bone marrow cavity area and average cortical bone thickness were measured from the photographs using the software Image Pro-plus 5.1 for Windows (Media Cybernetics, Silver Spring, MD, USA). Moreover, the cross-sectional moments of inertia (CSMI) of the failure sites were calculated using the methods for irregular cross-sections (Huang et al., 2008; Turner and Burr, 1993). Briefly, we divided the area of interest (AOI) into multiple 3x3 pixel squares. Then, we summarized the moments of inertia (MI) of those squares as a CSMI datum belonging to a single sample. Due to the size differences among specimens, a CSMI datum would be the summary from 400 ~ 600 individual MI in each cross-sectional image in the present study. The data were then converted to metric units. The CSMI of the failure site was calculated using the following equation:
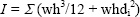 Furthermore, load-deformation data were transferred to stress-strain data using the following equations:
where is stress, σ is strain, c is the maximal distance from pixels to the line crossing the center of mass, F is the applied load (Nt), E is elastic modulus, d is the deformation (mm), and L is the span between the two support points of the bending fixture (mm).Because beam bending theory is only valid in the pre-yield region (Turner and Burr, 1993), we used the stress-strain curve to calculate yield stress, yield toughness and elastic modulus (Young’s modulus) as parameters of intrinsic (tissue-level) mechanical properties. Yield load and yield stress were determined with the 0.2%-offset method described in previous studies (Huang et al., 2008; Turner and Burr, 1993).
Dynamic histomorphometrySubsequent to MMA embedding, a cross-sectional cut was made near the fracture site of each femoral sample using a diamond blade low-speed cutter (S150, PlusOver Co. Ltd., Kaohsiung, Taiwan). All sections were ground to a consistent thickness of 100 ± 5 (µm) and photographed with a digital camera (COOLPIX 4500, Nikon, Japan) under a fluorescent light microscope ( 25). Dynamic histomorphometric parameters were measured in periosteal surfaces and endocortical surfaces according to Parfitt’s methods (Parfitt et al., 1987). All measurements were done by a trained investigator who was blinded to the original sample number. In the current study, we calculated percentage of mineralization surface (mineral surface/bone surface, MS/BS, %), mineral apposition rate (MAR, µm/day), and bone formation rate (BFR/BS, µm3 /µm2 /year) using the formulas below:
Statistical analysisThe data are presented as mean ± standard deviation (SD). For the data of BW and GRF, 2-way (time group) ANOVA was used to compare the differences among the 3 groups and 2 landing groups, respectively. For the data collected from serial analysis of bone tissues, one-way analysis of variance (ANOVA) was adopted to compare the differences among groups. When significant levels (p < 0.05) were revealed, pair-wise comparisons between groups were made using the Fisher’s least significant difference (LSD) method. Statistical analysis software, SPSS (14.0 version, SPSS, Chicago, IL), was adopted for processing the data in the present study.
Body weightWith the main effect of time, animals significantly increased in body weight throughout the experimental period (Figure 1). Beginning on day two, total average body weight values were significantly higher than those on day 0 . Body weight showed no difference between groups.
Ground reaction forceWe compared average hind-limb GRF values on the 1st day and 5th day in the two landing groups. For the main effect of time, the GRF of the groups on the 5th day showed a significant decrease as compared to the data of the 1st day (Figure 2). No difference was shown between the two landing groups.
Biomechanical analysesIn extrinsic (whole bone) mechanical tests, no significant difference was revealed in pre-yield mechanical behaviors, whereas the two landing groups had significantly lower femoral post-yield and maximal energy values when compared to the CON group (p < 0.05, Table 1). Additionally, the femora of the two landing groups were nearly significantly lower in energy to fracture load than those of the CON group (p = 0.057). No significant difference was revealed in intrinsic (tissue-level) mechanical measurements.
Tissue sizes and weightsCortical area and cortical thickness of femoral cross-sections were significantly lower in the exercise groups than in the CON group (p < 0.05, Table 2). In addition, total cross-section area was significantly lower in the L30 group than in the CON group. No significant difference was shown in weight measurements or in femoral length.
Dynamic histomorphometrySix samples were withdrawn due to a lack of two fluorescence labels, which included 2, 1 and 3 samples from the CON, L10 and L30 groups, respectively (Table 3). No significant difference was found in regions of periosteal surfaces and endocortical surfaces.
The present study preliminarily verified a decrease in extrinsic mechanical properties and cross-sectional size-related measurements of hind-limb bones following a short-term free-fall landing program. Such a compromise in tissue material might be due to a latency period in bone’s adaptation to external mechanical stimulus. Short-term free-fall landing caused a minor compromised bone tissueLooking at previous animal studies, our work provided a relatively confirmatory, rather than a de novo, finding. Results resembling our findings have been found using relatively basic research models. For instance, after a short-term axial loading period, rodents’ ulnae revealed decreased whole bone maximal strength, bending energy, and stiffness, as well as intrinsic bone mechanical properties (e.g. Young’s modulus and stress) (Chen et al., 2008; Hsieh and Silva, 2002). In addition to changes in cortical bone material, extremity bones subjected to short period of axial compression loading revealed a loss of trabecular tissue (Brodt and Silva, 2010) and reduced longitudinal growth (Ohashi et al., 2002) in murine. Compared to those apparatus-generated loading models, the current mechanical loading protocol caused a relatively minor compromise in bone material. After a 5-day free fall landing training, femora revealed reductions in post-yield and maximal energy absorption without significant changes in other extrinsic strength or intrinsic mechanical properties (Table 1). In addition, free-fall landing caused some lower values in the region of cortical bone (e.g. cortical area and thickness) without other significant differences in whole tissue weights (e.g. WW and FFDW) or linear growth of femora (Table 2). Contrary to former short- and long-term exercise studies (Huang et al., 2008; Lin et al., 2011; Wallace et al., 2007; 2009), which verified enhanced post-yield behavior of growing bone in young rodents, the current study reveals a reduced post-yield and maximal energy dissipation in the short-term free-fall landing groups (Table 1). Since the maximal energy value was composed of yield energy, and energy dissipated between yield load and maximal load, the differences between groups were caused primarily by the post-yield part of maximal energy. It has been suggested that post-yield behavior determines tissue toughness (Courtney et al., 1996), which is negatively correlated with microdamage accumulation (Leng et al., 2009). It has also been reported that excessive loads would cause microdamages (Verborgt et al., 2000). Post-yield behaviors are also associated with the integration of the extracellular matrix (ECM) (e.g. the content and the interaction among collagen and enzymatic/non-enzymatic crosslinks) (Garnero et al., 2006; Nyman et al., 2007). Bone tissues with higher post-yield energy absorption would be facilitated by highly organized collagen fiber (Burr, 2002; Skedros et al., 2006). Whether the changes in post-yield behavior in this study were associated with the accumulation of microdamage or the integration of ECM is worthy of further investigation. One reason that the current study showed decreased post-yield energy in hind-limb bones, which is contrary to previous findings in exercise related studies (3~8 weeks running training period) (Huang et al., 2008; Wallace et al., 2007; 2009), might be because of the relatively short period of our free-fall landing training. In the studies of Wallace et al., after 21-days of treadmill running, tibiae of male mice showed increased post-yield deformation, while pre-yield deformation decreased (Wallace et al., 2007; 2009). Two weeks after the 21-day treadmill running program, long bone tissues of mice showed advances in Young’s modulus and yield stress without showing differences in cross-sectional measurements, implying an enhancement in the quality of ECM (Wallace et al., 2009). Moreover, after eight weeks of running training, femoral bone also showed significantly higher post- yield bending energy (Huang et al., 2008). When compared to hind-limbs, forelimbs seem to respond to similar free-fall landing faster and/or better as shown by an increased post-yield energy in the same experiment (Lin et al., 2011). According to the GRF values, hind-limbs (10~15 times BW) were subjected to a higher average GRF than were forelimbs (4~5 times BW) (Lin et al., 2011), which might generate different levels of strain magnitude or strain rate on bone tissue (Edwards et al., 2009). Though external load-generated strain magnitude or strain rate on bone tissue is proportionally related to bone formation activity (Cullen et al., 2001; Torrance et al., 1994; Turner, 1998), osteoclastogenesis can be initiated by high strain magnitude or high compression load (Nozaki et al. , 2010; Xu et al., 2012). Overload-induced osteocyte apoptosis would also enhance the recruitment of osteoclasts to remove compromised tissue (Robling et al., 2006; Verborgt et al., 2000). Further study is needed to clarify whether the non-significant difference in BFR in the current study is due to a combined effect from higher osteoclastogenesis. In the current study, without a baseline control group, we are not able to determine whether compromised bone material is due to a transient suppression or an absolute decline in growth. Nevertheless, such compromises in bone material and size-related measurements are recoverable and might be due to a latency period in bone’s adaptation to external mechanical stimuli. As shown in previous studies, after an 8-day compressive loading protocol, male Sprague Dawley (SD) rats revealed a significant decrease in the longitudinal mineralization rate (LMR) of the ulnar growth plate (Ohashi et al., 2002). Seven days after loading regimes, the reduced LMR had been recovered in groups with lower loading levels (Ohashi et al., 2002). In another time serial study, ulnae of female rats were subjected to a single-bout fatigue loading. It was then verified that indexes of bone mechanical properties (e.g. whole bone maximal bending load and stiffness) reduced, recovered and then increased throughout the following 18-day recovery period (Hsieh and Silva, 2002). Moreover, according to the preliminary results of our latest time-serial experiment (data not shown), we duplicated the compromised phenomena in size-related measurements as well as biomechanical properties in rodents’ long bones after the 1st week of a free-fall landing protocol. Those compromised indexes recovered and then increased as compared to an age-matched control group after 2, 4 and 8 weeks of free-fall landing training. Thus, the lower post-yield energy of the two landing groups suggests that the bone tissue is undergoing remodeling caused by free-fall landing and not in an optimal state.
Study conditions, clinical applications and possible limitationsRegarding the rationality of the free-fall landing model, the animals in the current study should be subjected to a mechanical stress within a physiological range comparable to that of high-impact physical activities in humans. According to the GRF measurements, each forelimb had to withstand 4-5 times BW when landing (Lin et al., 2011), while a single hind-limb simultaneously sustained an impact at a level ~10-15 times BW. In everyday life, exercise with a 10~15-time BW GRF frequently occurs on the athletic field or in military training. For instance, triple jumping performed by elite athletes produces an average maximal GRF of 17.3-times BW (Heinonen et al., 2001). The lower extremities of young gymnasts sustain GRFs of 3.7~10.4 times BW when performing various gymnastics maneuvers (Daly et al., 1999). Even a simple drop jump from a height of 40cm~80cm causes 4.7~5.8 times BW of GRF (Viitasalo et al., 1998). In addition, a parachute landing generates 6.1~13.7 times BW of GRF with descent velocity (Whitting et al., 2007). Regarding the applicability of the current study to humans, it is possible that similarly compromised bone material could also occur during the initial period of a sport or military training season. Further investigation to determine whether an increase in landing height or number would cause more serious impairments and mimic the stress fractures occurring in young gymnasts (O'Kane et al., 2011), military trainees (Beck et al., 2000) and athletic recruits (Fredericson et al., 2006) would be valuable. It is possible that the procedures of free-fall landing might induce a stress-related response and thus negatively impact the bone materials. However, according to a former stress-related study, a comprehensive negative bone turnover and body weight loss would have occurred had the experimental animals been subjected to a chronic mild stress (Yirmiya et al., 2006). The compromised bone material shown in this study is more likely a local response of femora to free-fall landing impact. As mentioned above, a similar free-fall landing protocol induced a higher bone formation rate in ulnae (Lin et al., 2011), suggesting that animals did not suffer from a comprehensive negative bone turnover. Though related stress hormone analyses were not available in this study, the fact that there was no difference in body weight or bone length among groups throughout the experimental period indirectly suggests that the animals were free from sustained mental stress and hormone disorders. It is possible that a transient psychological stress caused by being handled or free-falling landing occurred during the daily training period; the related negative impact on bone metabolism should be limited.
In summary, data from our study indicate that short-term high-impact exercise seems to somewhat compromise bone tissue. According to the previous related studies mentioned above, such a phenomenon might represent a transient period in bone response to external mechanical loading, which needs to be clarified in the future. Studies using multiple time points and consistent animal species will be valuable for further exposing the procedures and/or related mechanisms. |
| AUTHOR BIOGRAPHY |
|
 |
Hsin-Shih Lin |
| Employment: National Taiwan Normal University & National Cheng Kung University (Taiwan), Ph.D. graduate student |
| Degree: MSc |
| Research interests: Exercise and bone metabolism |
| E-mail: 897300180@ntnu.edu.tw |
| |
 |
Tsang-Hai Huang |
| Employment: National Cheng Kung University (Taiwan), Associate Professor |
| Degree: PhD |
| Research interests: Exercise and bone metabolism |
| E-mail: tsanghai@mail.ncku.edu.tw |
| |
 |
Ho-Seng Wang |
| Employment: National Taiwan Normal University (Taiwan), Associate Professor |
| Degree: PhD |
| Research interests: Exercise physiology |
| E-mail: t08019@ntnu.edu.tw |
| |
 |
Shih-Wei Mao |
| Employment: R.O.C. Military Academy (Taiwan), Associate Professor |
| Degree: PhD |
| Research interests: Material Science |
| E-mail: swmao@mail.cma.edu.tw |
| |
 |
Yuh-Shiou Tai |
| Employment: R.O.C. Military Academy (Taiwan), Professor |
| Degree: PhD |
| Research interests: Material Science |
| E-mail: ystai@cc.cma.edu.tw |
| |
 |
Hung-Ta Chiu |
| Employment: National Cheng Kung University (Taiwan), Associate Professor |
| Degree: PhD |
| Research interests: Sports Biomechanics |
| E-mail: htchiu@mail.ncku.edu.tw |
| |
 |
Kuang-You B. Cheng |
| Employment: National Cheng Kung University (Taiwan), Associate Professor |
| Degree: PhD |
| Research interests: Sports Biomechanics |
| E-mail: kybcheng@mail.ncku.edu.tw |
| |
 |
Rong-Sen Yang |
| Employment: National Taiwan University Hospital (Taiwan), Professor |
| Degree: PhD |
| Research interests: Orthopaedics, osteoporosis & bone cancer |
| E-mail: rsyang@ntuh.gov.tw |
| |
|
| |
| REFERENCES |
 Beck T.J., Ruff C.B., Shaffer R.A., Betsinger K., Trone D.W., Brodine S.K (2000) Stress fracture in military recruits: gender differences in muscle and bone susceptibility factors. Bone 27, 437-444. |
 Brodt M.D., Silva M.J (2010) Aged mice have enhanced endocortical response and normal periosteal response compared with young-adult mice following 1 week of axial tibial compression. Journal of Bone and Mineral Research 25, 2006-2015. |
 Burr D.B (2002) The contribution of the organic matrix to bone’s material properties. Bone 31, 8-11. |
 Chen X.Y., Zhang X.Z., Guo Y., Li R.X., Lin J.J., Wei Y. (2008) Clinical Biomechanics. |
 Courtney A.C., Hayes W.C., Gibson L.J (1996) Age-related differences in post-yield damage in human cortical bone. Experiment and model. Journal of Biomechanics 29, 1463-1471. |
 Cullen D.M., Smith R.T., Akhter M.P (2001) Bone-loading response varies with strain magnitude and cycle number. Journal of Applied Physiology 91, 1971-1976. |
 Daly R.M (2007) The effect of exercise on bone mass and structural geometry during growth. Medicine and Sport Science 51, 33-49. |
 Daly R.M., Rich P.A., Klein R., Bass S (1999) Effects of high-impact exercise on ultrasonic and biochemical indices of skeletal status: A prospective study in young male gymnasts. Journal of Bone and Mineral Research 14, 1222-1230. |
 Edwards W.B., Ward E.D., Meardon S.A., Derrick T.R (2009) The use of external transducers for estimating bone strain at the distal tibia during impact activity. Journal of Biomechanical Engineering 131, 051009-. |
 Fredericson M., Jennings F., Beaulieu C., Matheson G.O (2006) Stress fractures in athletes. Topics in Magnetic Resonance Imaging 17, 309-325. |
 Garnero P., Borel O., Gineyts E., Duboeuf F., Solberg H., Bouxsein M.L., Christiansen C., Delmas P.D (2006) Extracellular post-translational modifications of collagen are major determinants of biomechanical properties of fetal bovine cortical bone. Bone 38, 300-309. |
 Harrast M.A., Colonno D (2010) Stress fractures in runners. Clinics in Sports Medicine 29, 399-416. |
 Heinonen A., Sievanen H., Kyrolainen H., Perttunen J., Kannus P (2001) Mineral mass, size, and estimated mechanical strength of triple jumpers’ lower limb. Bone 29, 279-285. |
 Hind K., Burrows M (2007) Weight-bearing exercise and bone mineral accrual in children and adolescents: a review of controlled trials. Bone 40, 14-27. |
 Honda A., Umemura Y., Nagasawa S (2001) Effect of high-impact and low-repetition training on bones in ovariectomized rats. Journal of Bone and Mineral Research 16, 1688-1693. |
 Hsieh Y.F., Silva M.J (2002) In vivo fatigue loading of the rat ulna induces both bone formation and resorption and leads to time-related changes in bone mechanical properties and density. Journal of Orthopaedic Research 20, 764-771. |
 Huang T.H., Chang F.L., Lin S.C., Liu S.H., Hsieh S.S., Yang R.S (2008) Endurance treadmill running training benefits the biomaterial quality of bone in growing male Wistar rats. Journal of Bone and Mineral Metabolism 26, 350-357. |
 Huang T.H., Lin S.C., Chang F.L., Hsieh S.S., Liu S.H., Yang R.S (2003) Effects of different exercise modes on mineralization, structure, and biomechanical properties of growing bone. Journal of Applied Physiology 95, 300-307. |
 Judex S., Zernicke R.F (2000) High-impact exercise and growing bone: relation between high strain rates and enhanced bone formation. Journal of Applied Physiology 88, 2183-2191. |
 Karlsson M.K (2007) Does exercise during growth prevent fractures in later life?. Medicine and Sport Science 51, 121-136. |
 Kato T., Yamashita T., Mizutani S., Honda A., Matumoto M., Umemura Y (2009) Adolescent exercise associated with long-term superior measures of bone geometry: a cross-sectional DXA and MRI study. British Journal of Sports Medicine 43, 932-935. |
 Leng H., Dong X.N., Wang X (2009) Progressive post-yield behavior of human cortical bone in compression for middle-aged and elderly groups. Journal of Biomechanics 42, 491-497. |
 Lin H.S., Huang T.H., Mao S.W., Tai Y.S., Chiu H.T., Cheng K.Y.B., Yang R.S (2011) A short-term free-fall landing enhances bone formation and bone material properties. Journal of Mechanics in Medicine and Biology 11, 1125-1139. |
 Nagasawa S., Honda A., Sogo N., Umemura Y (2008) Effects of low-repetition jump exercise on osteogenic response in rats. Journal of Bone and Mineral Metabolism 26, 226-230. |
 Nozaki K., Kaku M., Yamashita Y., Yamauchi M., Miura H (2010) Effect of cyclic mechanical loading on osteoclast recruitment in periodontal tissue. Journal of Periodontal Research 45, 8-15. |
 Nyman J.S., Roy A., Tyler J.H., Acuna R.L., Gayle H.J., Wang X (2007) Age-related factors affecting the postyield energy dissipation of human cortical bone. Journal of Orthopaedic Research 25, 646-655. |
 O'Kane J.W., Levy M.R., Pietila K.E., Caine D.J., Schiff M.A (2011) Survey of injuries in Seattle area levels 4 to 10 female club gymnasts. Clinical Journal of Sport Medicine 21, 486-492. |
 Ohashi N., Robling A.G., Burr D.B., Turner C.H (2002) The effects of dynamic axial loading on the rat growth plate. Journal of Bone and Mineral Research 17, 284-292. |
 Parfitt A.M., Drezner M.K., Glorieux F.H., Kanis J.A., Malluche H., Meunier P.J., Ott S.M., Recker R.R (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. Journal of Bone and Mineral Research 2, 595-610. |
 Pitukcheewanont P., Punyasavatsut N., Feuille M (2010) Physical activity and bone health in children and adolescents. Pediatric Endocrinolgy Review 7, 275-282. |
 Robling A.G., Castillo A.B., Turner C.H (2006) Biomechanical and molecular regulation of bone remodeling. Annual Review of Biomedical Engineering 8, 455-498. |
 Scerpella T.A., Davenport M., Morganti C.M., Kanaley J.A., Johnson L.M (2003) Dose related association of impact activity and bone mineral density in pre-pubertal girls. Calcified Tissue International 72, 24-31. |
 Skedros J.G., Dayton M.R., Sybrowsky C.L., Bloebaum R.D., Bachus K.N (2006) The influence of collagen fiber orientation and other histocompositional characteristics on the mechanical properties of equine cortical bone. Journal of Experimental Biology 209, 3025-3042. |
 Torrance A.G., Mosley J.R., Suswillo R.F., Lanyon L.E (1994) Noninvasive loading of the rat ulna in vivo induces a strain-related modeling response uncomplicated by trauma or periostal pressure. Calcified Tissue International 54, 241-247. |
 Tran Van P.T., Vignery A., Baron R (1982) Cellular kinetics of the bone remodeling sequence in the rat. Anatomical Record 202, 445-451. |
 Turner C.H (1998) Three rules for bone adaptation to mechanical stimuli. Bone 23, 399-407. |
 Turner C.H., Burr D.B (1993) Basic biomechanical measurements of bone: a tutorial. Bone 14, 595-608. |
 Umemura Y., Ishiko T., Yamauchi T., Kurono M., Mashiko S (1997) Five jumps per day increase bone mass and breaking force in rats. Journal of Bone and Mineral Research 12, 1480-1485. |
 Umemura Y., Nagasawa S., Honda A., Singh R (2008) High-impact exercise frequency per week or day for osteogenic response in rats. Journal of Bone and Mineral Metabolism 26, 456-460. |
 Verborgt O., Gibson G.J., Schaffler M.B (2000) Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. Journal of Bone and Mineral Research 15, 60-67. |
 Viitasalo J.T., Salo A., Lahtinen J (1998) Neuromuscular functioning of athletes and non-athletes in the drop jump. European Journal of Applied Physiology and Occupational Physiology 78, 432-440. |
 Wallace J.M., Rajachar R.M., Allen M.R., Bloomfield S.A., Robey P.G., Young M.F., Kohn D.H (2007) Exercise-induced changes in the cortical bone of growing mice are bone- and gender-specific. Bone 40, 1120-1127. |
 Wallace J.M., Ron M.S., Kohn D.H (2009) Short-term exercise in mice increases tibial post-yield mechanical properties while two weeks of latency following exercise increases tissue-level strength. Calcified Tissue International 84, 297-304. |
 Warren M.P., Chua A.T (2008) Exercise-induced amenorrhea and bone health in the adolescent athlete. Annals of New York Academic Science 1135, 244-252. |
 Welch J.M., Turner C.H., Devareddy L., Arjmandi B.H., Weaver C.M (2008) High impact exercise is more beneficial than dietary calcium for building bone strength in the growing rat skeleton. Bone 42, 660-668. |
 Welch J.M., Wade J.A., Hillberry B.M., Weaver C.M (2009) Force platform for rats measures fore and hind forces concurrently. Journal of Biomechanics 42, 2734-2738. |
 Welch J.M., Weaver C.M., Turner C.H (2004) Adaptations to free-fall impact are different in the shafts and bone ends of rat forelimbs. Journal of Applied Physiology 97, 1859-1865. |
 Whitting J.W., Steele J.R., Jaffrey M.A., Munro B.J (2007) Parachute landing fall characteristics at three realistic vertical descent velocities. Aviation Space and Environmental Medicine 78, 1135-1142. |
 Xu X.Y., Guo C., Yan Y.X., Guo Y., Li R.X., Song M., Zhang X.Z (2012) Differential effects of mechanical strain on osteoclastogenesis and osteoclast-related gene expression in RAW264.7 cells. Molecular Medicine Reports 6, 409-415. |
 Yirmiya R., Goshen I., Bajayo A., Kreisel T., Feldman S., Tam J., Trembovler V., Csernus V., Shohami E., Bab I (2006) Depression induces bone loss through stimulation of the sympathetic nervous system. Proceedings of the National Academy of Sciences of the United States of America 103, 16876-16881. |
|
| |
|
|
|
|

